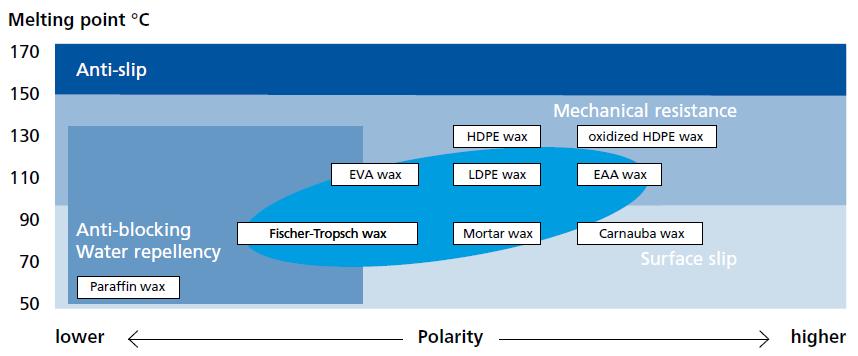
What Is Wax ?
Wax Definition
Wax is a broad term used to describe a general group of organic compounds. Early on, “wax” was often used as a synonym for “beeswax.” Later on, other natural materials were discovered that also showed wax-like properties and in the 20th century synthetic waxes became available. There is no generally accepted definition of waxes. A chemical description is not very meaningful, because the involved chemistries can be very diverse and not helpful in distinguishing waxes from non – wax materials.
Wax Properties
Physical and technical properties are more suitable as definitions. Among those properties are:
• Waxes are solids with a melting point above 100 °F (typically between 120 °F and 320 °F)
• They have a low melt viscosity (not more than 10 Pa·s at 20 °F above the melting point)
• They melt without decomposition
• They can be polished under slight pressure
The differentiation between waxes and organic polymers is not clear in all cases, e.g., polytetrafluoroethylene (PTFE) is often classified as a wax, but by definition it is not a wax because it has no melting point.
Wax Properties
Wax Types
Paraffin and Microcrystalline waxes are derived from petroleum. They are easy to recover and offer a wide range of physical properties that can often be tailored by refining processes. Most producers offer two distinct types of petroleum waxes: paraffins, which are distinguished by large, well formed crystals; and microcrystallines, which are higher melting waxes with small, irregular crystals. Microcrystalline wax contains substantial proportions of branched and cyclic saturated hydrocarbons in addition to normal alkanes.
Some producers also sell “intermediate” wax, in which the boiling range is cut where the transition in crystal size and structure occur. Petroleum wax producers also characterize wax by degree of refinement; fully refined paraffin has oil content generally less than 0.5%, and fully-refined micro-crystalline less than 3%. “Slack wax,” precursors to the fully refined versions in either case, would have oil content above 3%, and as high as 35% by weight. Paraffin wax produced from petroleum is essentially a pure mixture of normal and iso-alkanes without the esters, acids, etc. found in the animal and vegetable-based waxes.
Synthetic waxes have entered the wax market in the past 50 years. Polyethylene waxes are low molecular weight polyethylenes (less than 10,000 Mn) having wax-like properties made by either high-pressure or low-pressure (Zeigler-type catalyst) polymerization. All such waxes have the same basic structure, but the various production processes yield products with distinctly different properties, and these have a major impact on the use of products. Products from one manufacturer may satisfy one particular application, while product from a similar process will not work well.
Fischer-Tropsch (FT) wax is a synthetic wax produced by the polymerization of carbon monoxide under high pressure, a technology used in the emerging natural Gas to Liquid (GTL) projects. The hydrocarbon product of FT reaction is distilled to separate the mix into fuels products and waxes with melting points ranging from about 45 – 106ºC. Currently FT waxes are commercially produced in South Africa and Malaysia. It is estimated that the overall synthetic wax consumption in North America in 2010 was 420 million lbs., of which FT wax accounts for about 195 million lbs.
Alpha olefin waxes are synthetically derived from ethylene via a Ziegler-Natta catalyst. The process results in a Schulz-Flory distribution of alpha olefins ranging from C4 through C30+. These are distilled into the individual carbon fractions or carbon fraction blends. Due to the high melting points of the waxes, C20 and higher carbon numbers are fractionated into blends. Because of the linear double bond present in normal alpha olefins, these waxes can be functionalized or reacted to create other derivatives. They can also be used for their physical properties such as hardness and melting point. End uses for alpha olefin waxes include lube oil additives, PVC lubricants, candles, oilfield chemicals and personal care applications.
Montan wax is derived by solvent extraction of lignite. The earliest production of montan wax on a commercial scale was in Germany during the latter half of the nineteenth century. Germany continues to lead the world in production of montan wax; although some montan wax is produced in the United States from the Ione lignite bed in California. The composition of montan wax varies geographically with production, but includes varying amounts of wax, resin and asphalt.
Other mineral waxes include peat waxes, ozokerite and ceresin waxes.
Beeswax has been traded for over 2,000 years and references to “wax” before the 19th century typically meant beeswax. Yellow beeswax is secreted by bees to build honeycombs; the empty comb is melted in boiling water to recover the wax.
Other animal-based waxes include lanolin from the wool of sheep; ambergris produced in the intestines of sperm whales; and tallow from beef fat.
Carnauba wax is recovered from a variety of palm tree which grows almost exclusively in northeastern Brazil. Carnauba wax forms on the fronds of the trees and is recovered by cutting and drying the fronds, then mechanically removing the wax. Impurities are removed from the wax by melting and filtering, or centrifuging.
Candelilla wax is harvested from shrubs grown in the Mexican states of Coahuila and Chihuahua and in Texas. The entire mature plant is uprooted and immersed in boiling water acidified with sulfuric acid; the wax floats to the surface for recovery.
Other vegetable-based waxes include Japan wax, produced on the berries of a small tree native to Japan and China; ouricury wax, obtained from the fronds of another type of palm tree growing in Brazil; rice-bran wax, extracted from crude rice bran; and jojoba, obtained from the seeds of the jojoba plant grown in parts of Costa Rica, Israel, Mexico and the United States, and soy wax which is produced by hydrogenated soybean oil.
Wax Additives
Waxes are solid materials and cannot be used directly in many applications. It is necessary to convert them into wax additives that are easy to handle and can be readily incorporated into all types of systems. Wax additives can be finely dispersed waxes in a liquid carrier (water or organic solvents) or micronized waxes in powder form. Micronized wax additives are ideal for solvent-free applications but they also can be easily stirred into liquid systems. Wax additives are formulations and may contain more than one type. Wax combinations are often used to create wax additives with unique features.
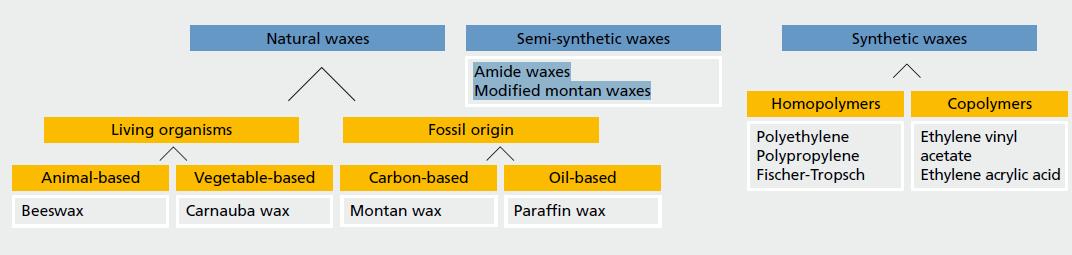
Origin of Waxes