caustic soda production process

Caustic Soda Production Process
Sodium hydroxide (chemical formula is NaOH) is an inorganic compound with following synonyms:
- Caustic soda
- Lye
- Sodium hydrate
NaOH widely used the inorganic industrial chemical.
NaOH molecular weight of 39.997 g/mol
Sodium hydroxide is contained ph of ~12-14
Caustic soda is available in two forms: Caustic soda lye and Caustic soda solid. The solid form of the caustic soda can be in the form of Caustic soda flakes or Caustic soda granules (Pearls).
The pure form of sodium hydroxide also available as:
- Sodium hydroxide pellets
- Sodium hydroxide flakes
- Sodium hydroxide granule
- Sodium hydroxide solution
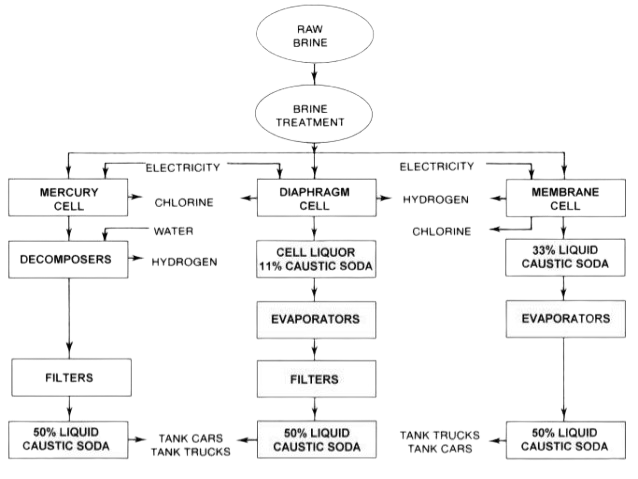
Sodium Hydroxide Solutions are produced by three different technologies:
- Mercury cells
- Membrane cells
- Diaphragm cells
Each of the above processes utilizes sodium chloride (NaCl) salt as the primary raw material. Electrolytic splitting of salt results in products like chlorine and sodium ion (Na+). In turn Na+ will react with water in the mercury cell to form Sodium hydroxide and Hydrogen as by-product.
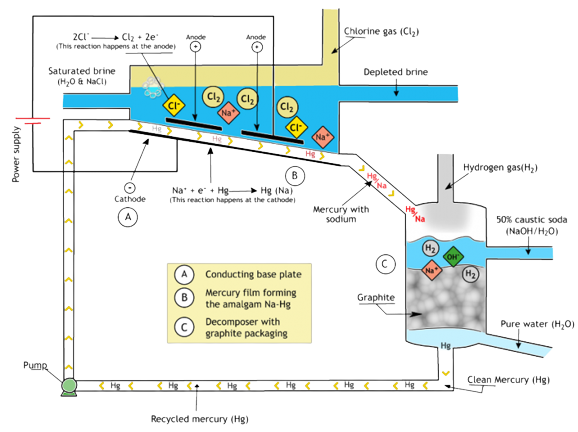
Mercury Cell
In the Mercury Cell Process saturated brine voyages down a steel trough roughly 15 meters in length and one meter wide between a streaming film of mercury (the cathode) and titanium plates (the anodes). Direct current is connected between the anode and cathode. Chlorine freed at the anodes gathers above the brine and is begun as a hot, wet and corrosive gas.
Sodium ions are released at the surface of the streaming mercury cathode, forming an amalgam of low concentration with the mercury, which streams out of the cell without reacting with the water or chlorine.
The mercury cell thus has two products
(i) Hot, wet chlorine
(ii) Sodium amalgam
The soda cell or decomposer is a cylindrical steel trough loaded with graphite balls or graphite electrodes. The sodium amalgam is passed, along with pure water, into the decomposer, where it reacts to transform Sodium hydroxide as a controlled 50 percent aqueous solution and hydrogen gas, liberating fee mercury, which is reused again to the electrolytic cell. The graphite provides a surface that expedites this reaction.
The following two types of reactions called brine cell and the soda cell respectively.
Brine Cell
2Cl = Cl2 + e
Na + e = Na
Na + Hg = Na/Hg
Soda Cell
2 Na/Hg + 2 H2O = 2NaOH + H2 + 2Hg
This method produces 71 percent of Sodium hydroxide.
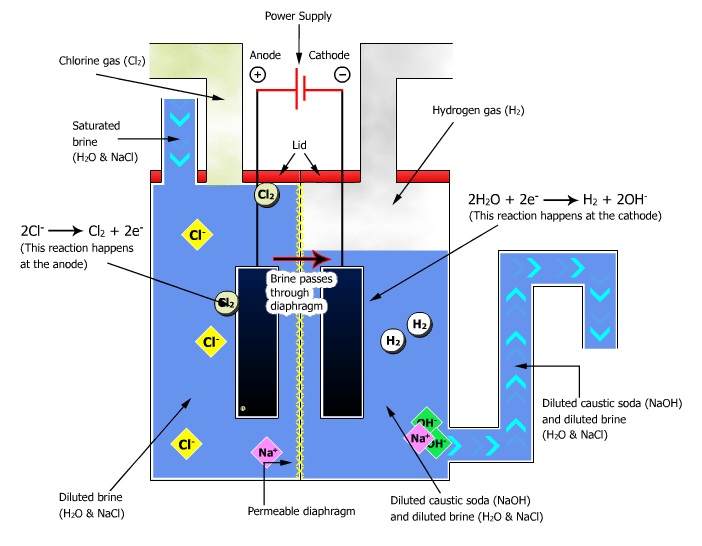
Diaphragm Cell
Diaphragm Cell process utilizes asbestos or alternate substitutes to asbestos, to separate the co-products Sodium Hydroxide (Caustic Soda) and Chlorine. The production of 50 percent NaOH occurs primarily outside of the electrolytic cell.
The diaphragm cell produces a very weak ‘cell liquor,’ that contains 12-14 percent, by weight, NaOH and the constant volume of NaCl salt. The cell liquor is subsequently evaporated in a three or four ‘effect’ evaporation method to a final nominal concentration of 50 percent NaOH by weight. The surplus salt is precipitated and filtered through the evaporation method for subsequent reuse/recycle. This method produces the lowest quality electrochemical NaOH solutions.
The quality considerations with respect to the diaphragm cell produced Caustic solutions include comparatively high salt, chlorates, carbonates, and sulfates. Salt, as NaCl, concentrations are typically 1.0 percent, with maximums ranging from 1.1 to 1.3 weight percent, counting on the producer.
The diaphragm cell created Caustic Soda (NaOH) is usually referred as Diaphragm Cell Grade. It is conjointly known as Commercial Grade, Technical Grade, and occasionally Technical Diaphragm or other similar combinations.
An additional ‘grade’ of Caustic Soda (NaOH) produced by the diaphragm cell method is the sublimate grade. The production of sublimate Grade involves the further evaporation of the 50 percent Diaphragm Grade NaOH solution to cut back the salt concentration. The higher concentration solution is then re-diluted to the 50% concentration that is commercially available as sublimate grade Caustic Soda.
Common uses include process and sewer water neutralization, textiles production, soaps and detergents, and aluminum production. These uses and applications typically can confer with the Caustic Soda as any of the varied grades.
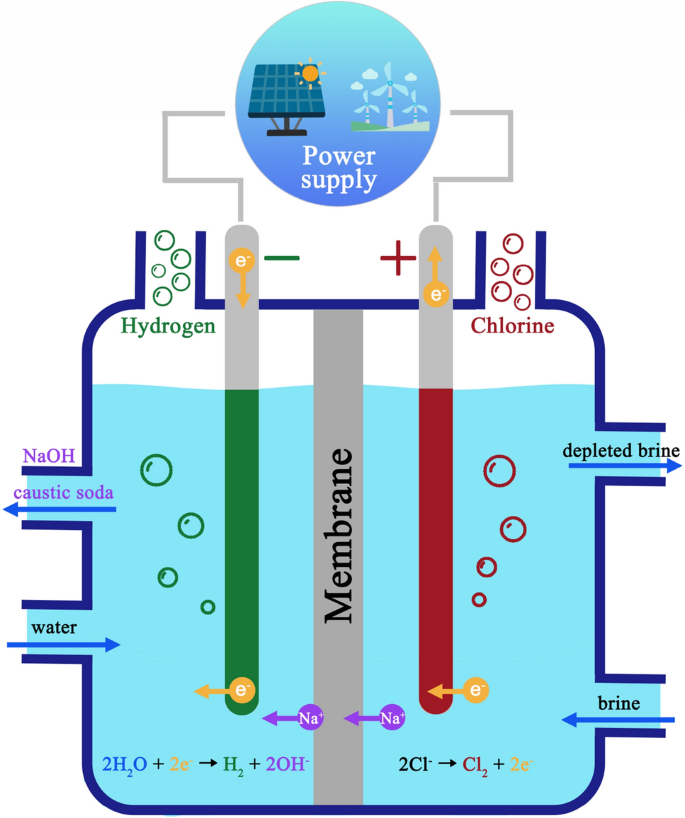
Membrane Cell
This method produces approximately 13 percent of Sodium Hydroxide. The membrane cell method utilizes a selective membrane that separates the Chlorine and Sodium ions. The membrane permits the Sodium ion to migrate across the membrane whereas keeping the Chlorine gas and salt (brine) solution in a compartment on the opposite facet of the membrane.
The Sodium ion is reacted with refined water as within the mercury cell to provide the Caustic Soda (NaOH). Evaporation is employed, as within the diaphragm method, to lift the concentration up to the nominal 50 weight percent solution. The salt concentrations are not targeted as considerably during this evaporation method attributable to the selective diffusion nature of the membranes as well as the reduced quantity of evaporation needed during this method as opposed to the diaphragm evaporation.
The Caustic Soda produced by the membrane cell process is most typically brought up as Membrane Grade. It conjointly contains a growing acceptance as a Rayon Grade product in all areas outside of rayon fiber production.